Neuramis Filler in London
Neuramis represents a sophisticated Korean-manufactured dermal filler designed with advanced cross-linked hyaluronic acid technology. Manufactured by Medytox Inc., this injectable addresses volume loss, facial contours, and age-related changes through precision application. The treatment typically requires 20-30 minutes, delivering immediate enhancement with continued improvement over subsequent weeks. Patients seeking balanced augmentation across multiple anatomical regions find this formulation particularly effective for achieving harmonious, natural-looking outcomes without excessive correction.




carrying out the procedure on the day of the appointment
Neuramis Pricing Overview in London
Treatment Area | Price Range | Syringes | Duration |
Lips | £295 – £395 | 0.5-1 | 9-12 months |
Cheeks | £395 – £650 | 1-2 | 12-18 months |
Nasolabial Folds | £295 – £395 | 0.5-1 | 9-12 months |
Under Eyes | £350 – £450 | 0.5-1 | 9-12 months |
Return patients receive 10% reduction on subsequent sessions when scheduled within recommended timeframes. Combination packages pairing Neuramis Deep with complementary aesthetic procedures offer 15% savings compared to individual pricing. Multi-area treatment plans spanning three or more zones qualify for customized rate structures based on total volume requirements.
Become an Aesthetic Model
We are currently inviting models to participate in our professional before-and-after photography programme for dermal filler procedures. This is not a training opportunity – all filler injections are performed exclusively by our experienced Aesthetic Practitioners with 7+ years of expertise in advanced injectable techniques, ensuring precise placement, natural results, and the highest safety standards.
This exclusive opportunity allows you to experience our advanced dermal filler treatments at a fraction of the standard cost whilst contributing to our website and practitioners’ social networks.
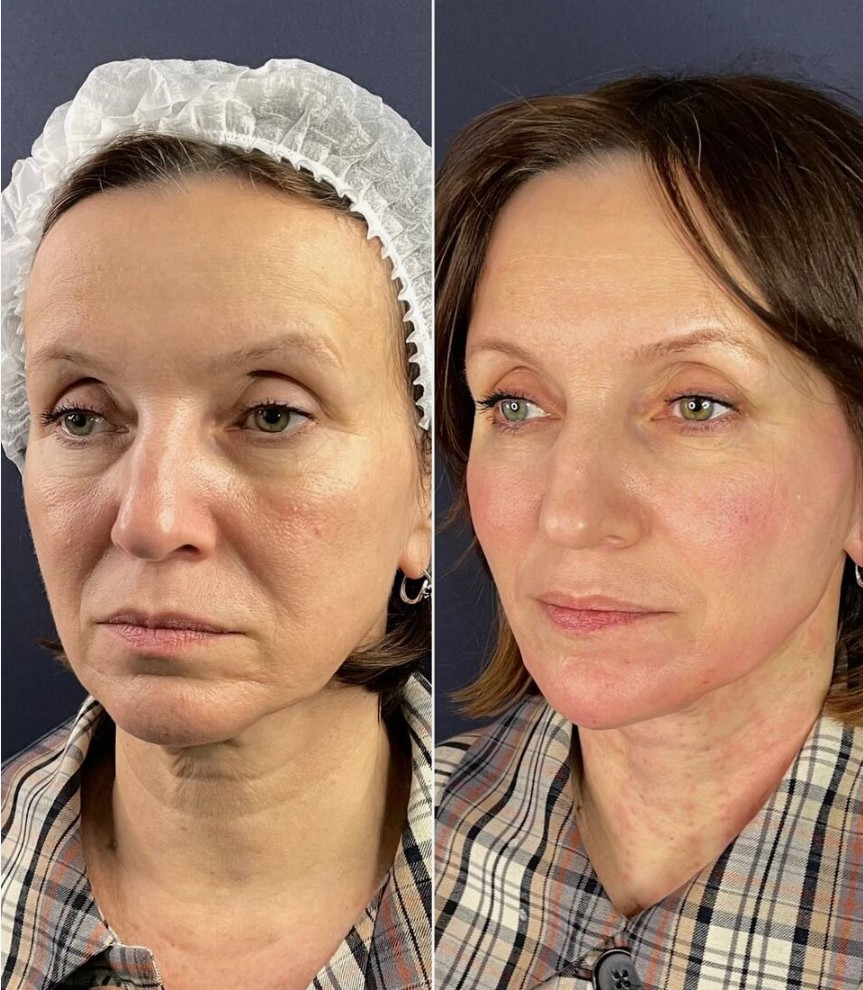
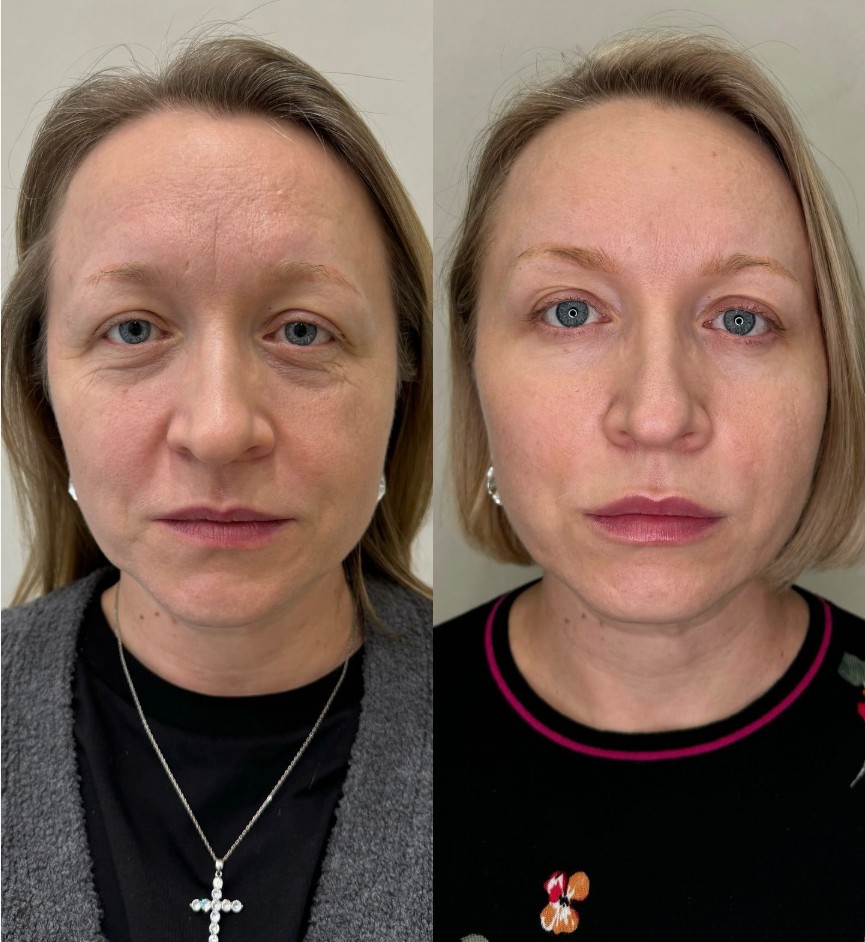
Before and After: Neuramis Results in London
Visible transformation manifests immediately following injection, with continued refinement developing over 72-120 hours as the hyaluronic acid fully integrates within tissue planes. Patients observe enhanced volume restoration, improved contour definition, and softening of established fold patterns. The formulation’s rheological properties enable smooth distribution throughout targeted areas, creating seamless transitions between treated and adjacent tissue. Maximum aesthetic benefit typically manifests within one week, presenting balanced enhancement that respects individual facial architecture rather than imposing artificial alteration.
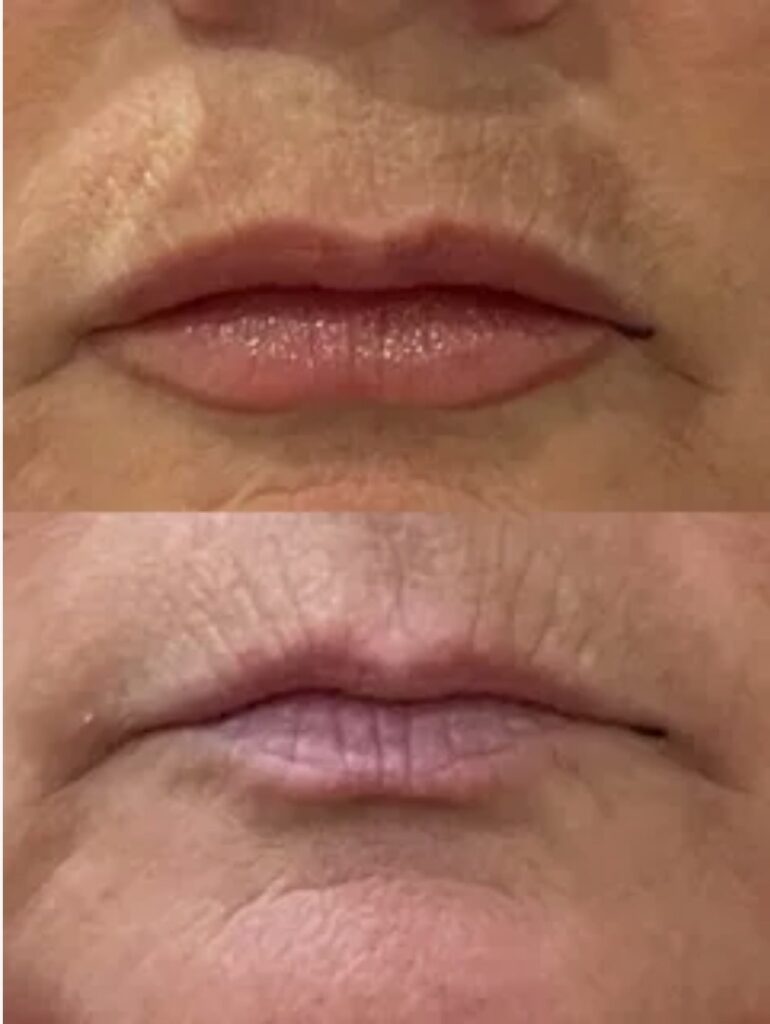
What is Neuramis?
Manufacturer and Origin
Medytox Inc., based in South Korea, produces Neuramis through pharmaceutical-grade manufacturing protocols established over two decades of biotechnology research. The company maintains ISO 13485 certification and operates under stringent quality management systems governing sterile production environments. This South Korean manufacturer has established distribution networks across 60 countries, reflecting international confidence in their production standards.
Composition and Molecular Structure
Each 1 ml syringe contains 20 mg/ml of cross-linked HA derived through bacterial fermentation, processed to achieve consistent molecular weight distribution. The formulation incorporates phosphate-buffered saline maintaining physiological pH 7.0, ensuring tissue compatibility upon injection. Neuramis Deep utilizes larger particle sizes (400-600 μm) compared to lighter formulations, providing structural support appropriate for mid-to-deep dermal placement. The addition of 0.3% lidocaine hydrochloride addresses procedural discomfort without compromising product stability.
Unique Technological Characteristics
The proprietary SHAPE™ (Stability through Hyaluronic Acid Persistence Enhancement) technology employed during manufacturing creates uniform cross-link density throughout the gel matrix. This consistency translates to predictable tissue integration and resistance to premature enzymatic degradation. The cross-linking ratio achieves optimal balance between cohesivity and malleability, allowing precise placement while preventing unwanted migration from injection sites. Particle homogeneity ensures smooth extrusion through fine-gauge needles without clogging or irregular flow.
Professional Selection Criteria
Aesthetic practitioners favor this Korean dermal filler for its reliable performance across diverse patient demographics and treatment objectives. The product’s moderate G-prime (elastic modulus) value positions it between soft tissue fillers and robust volumizers, offering versatility for addressing both dynamic lines and structural volume deficits. Clinicians appreciate the minimal inflammatory response profile and consistent resorption timeline, facilitating accurate treatment planning for maintenance sessions.
Regulatory Approvals
Neuramis holds CE marking under the European Medical Device Regulation (MDR), confirming compliance with safety and performance requirements for injectable medical devices. While FDA approval remains pending for the United States market, the product maintains authorization throughout the UK, European Union, and multiple Asian markets. Regular post-market surveillance data submission to regulatory bodies demonstrates ongoing commitment to pharmacovigilance standards.
Comparative Analysis
When evaluated against Restylane, Neuramis demonstrates comparable longevity but often presents lower pricing structure, making it cost-effective for patients requiring multiple treatment sessions. Compared to Juvéderm, the Korean formulation exhibits slightly firmer consistency, which certain injectors prefer for cheek augmentation and chin contouring applications. Against Belotero Balance, Neuramis Deep provides greater lifting capacity for deeper fold correction, though Belotero may offer superior superficial line integration. Each alternative presents distinct advantages depending on specific anatomical requirements and desired aesthetic endpoints.
The Neuramis Treatment Process: Step by Step
Initial Consultation and Facial Analysis
The practitioner conducts comprehensive facial assessment, photographing patients from multiple angles under standardized lighting conditions. Discussion encompasses aesthetic concerns, medical history, previous cosmetic interventions, and realistic outcome expectations. Three-dimensional facial analysis identifies volume deficits, asymmetries, and proportional relationships between facial thirds. This consultation phase typically extends 20-30 minutes, establishing customized injection strategies aligned with individual anatomy.
Pre-Treatment Preparation
Thorough cleansing removes makeup, oils, and surface contaminants from treatment zones using medical-grade antiseptic solution. Topical anesthetic cream (lidocaine 4-5%) may be applied 15-20 minutes before injection for patients with heightened sensitivity, though the product’s integrated lidocaine often provides adequate comfort. Strategic marking delineates injection points and vector directions, guided by anatomical landmarks and patient-specific concerns. Sterile technique preparation includes glove application and opening sealed product packaging in the patient’s presence, confirming authenticity.
Injection Technique and Depth
The practitioner employs 27G-30G needles or blunt-tip cannulas depending on anatomical location and tissue characteristics. For nasolabial folds, linear threading technique deposits product along the fold depth at the dermal-subcutaneous junction. Lip enhancement utilizes serial puncture or fanning methods, carefully avoiding vascular structures while building incremental volume. Cheek augmentation requires supraperiosteal or deep subcutaneous placement to achieve proper structural support. Each injection site receives gentle massage to encourage even distribution and prevent nodule formation.
Contouring and Refinement
Following initial injection, the practitioner molds treated areas through controlled external manipulation, ensuring smooth surface integration and symmetrical appearance. This contouring phase addresses any minor irregularities or areas requiring supplemental product. Patient feedback during this stage allows real-time adjustment to achieve desired aesthetic endpoints. The practitioner evaluates results from multiple angles, including profile view and dynamic facial expression, before considering the procedure complete.
Post-Treatment Protocol
Ice packs or cooling devices minimize immediate swelling and provide comfort during the first 15-20 minutes following injection. Patients receive detailed aftercare instructions covering activity restrictions, skincare modifications, and signs requiring clinical attention. Follow-up photography documents immediate post-treatment appearance for comparison at subsequent appointments. Most patients leave the clinic within 45-60 minutes of arrival, with mild erythema or swelling representing typical immediate responses.
Procedure Duration: 25-35 minutes
Recovery Period: Most patients resume normal activities within 24-48 hours, with residual swelling resolving over 3-7 days depending on treatment extent.
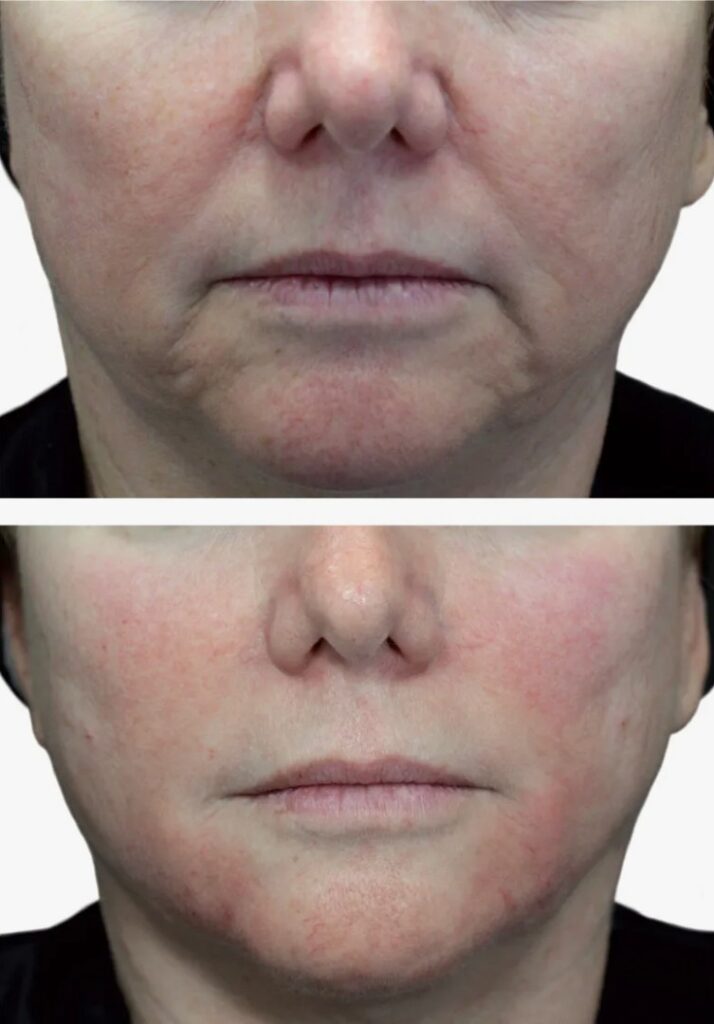
Benefits of Neuramis Filler
Natural-Looking Results
The formulation’s rheological properties enable integration within existing tissue architecture without creating visible edges or palpable irregularities. Unlike some high-cohesivity alternatives that may produce artificial fullness, this balanced consistency mimics native tissue texture. Patients report that friends and colleagues notice refreshed appearance without identifying specific interventions, reflecting successful aesthetic enhancement rather than obvious alteration.
Extended Duration
Clinical studies document efficacy persistence ranging from 9-18 months depending on treatment location, individual metabolism, and lifestyle factors. Deeper placements in cheek and chin areas demonstrate longest retention, while dynamic regions such as perioral lines may require retreatment at shorter intervals. This longevity reduces annual maintenance burden compared to shorter-acting formulations requiring quarterly visits.
Minimal Downtime Requirements
The vast majority of patients experience only transient erythema and mild swelling, permitting immediate return to work and social engagements. Bruising occurs in fewer than 20% of cases, typically resolving within 5-7 days with appropriate post-care measures. Unlike surgical interventions requiring weeks of recovery, this non-invasive approach fits seamlessly into busy professional schedules.
Anatomical Versatility
The Neuramis range encompasses multiple viscosities suitable for superficial lines, mid-dermal wrinkles, deep folds, and volumetric restoration. Practitioners can address multiple concerns during single sessions, treating tear troughs, lips, and cheeks with appropriate formulation selection. This versatility reduces total treatment time and allows comprehensive facial rejuvenation through coordinated injection strategies.
Established Safety Profile
Post-market surveillance data encompassing millions of syringes administered worldwide demonstrates low adverse event rates when proper injection technique is observed. The biocompatible nature of HA minimizes allergic reaction risk, as this molecule exists naturally in human dermis. Reversibility through hyaluronidase injection provides additional safety margin for unlikely complications or dissatisfaction with results.
Migration Resistance
The appropriate cross-link density prevents gel displacement from intended anatomical locations during normal facial movement and expression. This stability proves particularly important in mobile areas where poorly-formulated products might shift over time, creating asymmetry or unnatural contours. Patients maintain consistent appearance throughout the product lifespan without progressive distortion.
Compatibility with Complementary Procedures
Neuramis integrates effectively within comprehensive aesthetic treatment plans incorporating neurotoxin injections, laser resurfacing, microneedling, and chemical peels. The timing of combined treatments follows established protocols to optimize safety and results. Many patients schedule neurotoxin and filler sessions concurrently, maximizing aesthetic improvement while minimizing clinic visits.

Our Neuramis Treatment Options in London
Standard Volume Application
Single 1 ml syringe treatments address focused concerns such as isolated nasolabial fold correction or subtle lip enhancement. This entry-level option suits patients exploring injectable treatments for the first time or those maintaining previous results through periodic touch-ups. The practitioner concentrates product in primary concern areas while preserving natural facial balance.
Comprehensive Enhancement Protocol
Multi-syringe sessions utilizing 2-3 ml enable simultaneous treatment of multiple facial zones, creating harmonious rejuvenation across upper, mid, and lower face regions. This approach proves particularly effective for patients exhibiting generalized volume loss associated with natural aging processes. Strategic distribution across cheeks, temples, and jawline restores youthful contours without focal over-correction.
Combination Treatment Packages
Pairing Neuramis with complementary modalities amplifies overall aesthetic impact through synergistic mechanisms. Popular combinations include neurotoxin for dynamic wrinkle relaxation alongside volumetric restoration, or laser therapy for surface texture improvement coupled with structural support. These coordinated approaches address both superficial and deep-tissue concerns within unified treatment timelines.
Sequential Treatment Series
Some patients benefit from staged application over 2-3 appointments separated by 4-6 weeks, allowing gradual transformation that appears more natural to observers. This conservative strategy minimizes sudden change while permitting ongoing refinement based on evolving results. Sequential protocols prove especially valuable for significant volume replacement requirements exceeding what single sessions can safely accomplish.
Maintenance Refresh Sessions
Established patients returning for periodic enhancement receive streamlined appointments focusing on specific areas showing earliest resorption signs. These efficient visits preserve achieved results without requiring comprehensive retreatment, optimizing both time investment and cost efficiency. Maintenance intervals typically extend 9-15 months depending on initial treatment extent and individual factors.
Individualized Protocol Development
Every patient receives customized treatment design reflecting their unique anatomy, aesthetic goals, and practical considerations including budget and schedule constraints. Initial consultations explore various options, presenting clear information about expected outcomes, maintenance requirements, and total investment across different approaches. This transparent planning process ensures alignment between patient expectations and achievable results.
Why Choose Our London Clinic for Neuramis?
Practitioner Credentials and Expertise
Our clinical team maintains General Medical Council registration and holds advanced certification in aesthetic medicine through recognized training programs. Years of specialized experience in facial anatomy and injection techniques inform every treatment decision. Continuous professional development ensures familiarity with evolving protocols and emerging safety data within the injectable field.Product Authentication Guarantee
We source all supplies directly from authorized UK distributors, maintaining complete chain-of-custody documentation. Each syringe’s batch number is recorded in patient files, with lot-specific quality certificates available upon request. This commitment to authenticity eliminates counterfeit product risks that can compromise both safety and effectiveness.Results Assurance Policy
Patients dissatisfied with outcomes receive complimentary adjustment appointments within specified timeframes, ensuring satisfaction with final aesthetic results. While individual responses vary based on biological factors, our experienced team reliably achieves natural enhancement aligned with pre-treatment discussions. This guarantee reflects confidence in technical execution and product quality.Comprehensive Liability Coverage
Fully insured practice operations include professional indemnity coverage addressing unlikely complications arising from treatment. This financial protection provides peace of mind beyond basic clinical expertise, demonstrating organizational commitment to patient welfare. Emergency protocols and reversal agent availability further reinforce safety infrastructure.Bespoke Treatment Planning
Facial analysis considers not only isolated concerns but overall harmony, proportionality, and age-appropriate aesthetics. Treatment plans may recommend staged approaches, alternative modalities, or even deferral when interventions seem premature or inappropriate. This honest, patient-centered methodology prioritizes optimal outcomes over transaction volume.Structured Follow-Up Program
Post-treatment communication includes scheduled check-in contacts at 48 hours, one week, and four weeks to monitor healing progression and address any concerns. Patients receive direct practitioner contact information for questions arising between appointments. This ongoing support extends beyond procedure completion, fostering long-term treatment relationships.Neuramis vs Other Fillers: Comparison Chart
| Feature | Neuramis | Restylane | Juvéderm |
| Duration | 9-18 months | 9-18 months | 12-18 months |
| Price | £295-650 | £350-700 | £400-750 |
| Viscosity | Medium-High | Medium | High |
| Best For | Multi-area, folds, volume | Lines, lips, under eyes | Volume, contouring |
| Recovery Time | 1-3 days | 2-4 days | 2-5 days |
| Natural Feel | Yes | Yes | Yes |
Frequently Asked Questions About Neuramis
How long does Neuramis last?
Typical duration ranges from 9-18 months depending on treatment location, with deeper placements in structural areas demonstrating longest persistence. Individual metabolic rates, lifestyle factors including sun exposure and smoking, and dynamic facial movement patterns influence resorption timing. Most patients schedule maintenance between 10-14 months after initial treatment.
Is Neuramis safe?
Extensive clinical data and post-market surveillance confirm favorable safety profiles when administered by trained practitioners following proper protocols. The biocompatible HA composition minimizes immunogenic responses, with adverse events primarily limited to transient injection-site reactions. CE certification and widespread international use demonstrate regulatory confidence in product safety.
Can I combine Neuramis with other treatments?
Yes, strategic combination with neurotoxins, laser therapy, microneedling, and chemical peels creates comprehensive rejuvenation addressing multiple aging mechanisms simultaneously. Proper treatment sequencing ensures safety and optimal results. Practitioners typically schedule neurotoxin and filler during the same appointment, while ablative procedures require appropriate intervals before or after injection.
What are the side effects?
Common immediate responses include temporary swelling, redness, tenderness, and occasional bruising at injection sites, typically resolving within 3-7 days. Rare complications such as infection, vascular occlusion, or granuloma formation occur in fewer than 0.1% of cases when proper technique and anatomical knowledge guide treatment. Most patients experience only minor discomfort and minimal visible effects.
When will I see results?
Immediate volume enhancement becomes apparent upon treatment completion, with final aesthetic outcome developing over 5-7 days as swelling subsides and the product fully integrates. Some patients notice continued refinement for up to two weeks as tissue accommodation occurs. Photography comparison clearly documents transformation achieved through the procedure.
Can Neuramis be reversed?
Yes, hyaluronidase enzyme injections rapidly dissolve HA-based fillers when correction becomes necessary due to complications or dissatisfaction. This reversibility provides important safety margin distinguishing HA products from permanent alternatives. Complete resolution typically occurs within 24-48 hours following hyaluronidase administration.
How much Neuramis do I need?
Required volume varies significantly based on treatment objectives, anatomical baseline, and desired enhancement degree. Conservative approaches may utilize 0.5-1 ml for focused concerns, while comprehensive facial rejuvenation might require 3-5 ml distributed across multiple zones. Practitioners recommend starting conservatively, with additional product added during subsequent appointments if desired.
Is there downtime?
Most patients resume normal activities immediately, though strenuous exercise, excessive heat exposure, and alcohol consumption should be avoided for 24-48 hours. Visible swelling and redness typically resolve within 1-3 days, with makeup application permissible after 12-24 hours. Social engagements can generally be scheduled 2-3 days post-treatment to allow initial healing.
Does Neuramis work for men?
Absolutely. Male patients seek injectable treatments with increasing frequency, often preferring subtle enhancement that maintains masculine facial characteristics. Treatment approaches for men typically emphasize structural definition and volume restoration rather than softening, with injection strategies adapted to thicker skin and different aesthetic ideals.
What makes Neuramis different from other Korean fillers?
While multiple Korean manufacturers produce quality HA fillers, Neuramis distinguishes itself through the proprietary SHAPE™ cross-linking technology creating uniform particle structure. This consistency enables predictable performance and smooth tissue integration. Medytox’s established reputation and extensive international distribution network provide additional confidence in manufacturing standards and quality control.